Understanding the concept of polar versus non-polar molecules is fundamental in chemistry and plays a crucial role in various scientific disciplines. Whether you're studying chemical reactions, material science, or biological processes, molecular polarity determines how substances interact with one another. By delving into the differences between polar and non-polar molecules, we can better comprehend the behavior of compounds in different environments.
Chemistry is the study of matter, and one of the most fascinating aspects of this field is how molecules interact with one another. At the heart of these interactions lies molecular polarity, a concept that explains why some substances dissolve in water while others do not. This article will explore the intricacies of polar and non-polar molecules, providing you with the tools to grasp their importance in everyday life and advanced scientific research.
As we navigate through this guide, you'll discover the definitions of polar and non-polar molecules, their properties, and the factors influencing molecular polarity. Moreover, we'll examine real-world applications of these concepts and how they impact industries such as pharmaceuticals, agriculture, and environmental science. Let’s begin by breaking down the basics of polar vs non-polar molecules.
Read also:Brooke Sweeten The Rising Star Redefining Success In The Entertainment World
Table of Contents
- Definition of Polar and Non-Polar Molecules
- Understanding Dipole Moment in Polar Molecules
- The Role of Molecular Geometry in Polarity
- Electronegativity and Its Impact on Polarity
- Examples of Polar and Non-Polar Substances
- Real-World Applications of Polar and Non-Polar Molecules
- Comparison Between Polar and Non-Polar Molecules
- Solubility and Polarity: How They Relate
- Methods for Testing Molecular Polarity
- Conclusion: Why Understanding Polarity Matters
Definition of Polar and Non-Polar Molecules
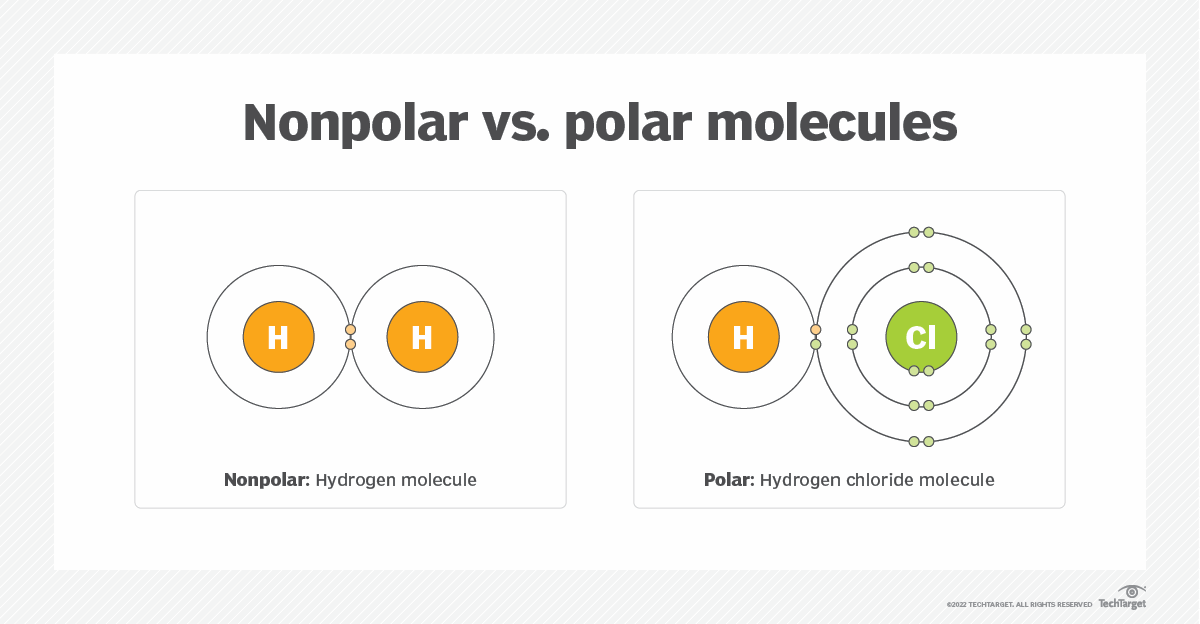
In chemistry, the term "polar" refers to molecules that have an uneven distribution of electrical charge, while "non-polar" describes molecules with an even distribution of charge. This distinction is critical because it influences how molecules interact with each other and their surroundings.
Polar molecules possess a partial positive charge on one end and a partial negative charge on the other, creating a dipole. This uneven charge distribution arises due to differences in electronegativity between the atoms within the molecule. On the other hand, non-polar molecules exhibit no such charge separation, as the electrons are shared equally among the atoms.
Characteristics of Polar Molecules
Polar molecules often form strong intermolecular forces, such as hydrogen bonds, which result in higher boiling and melting points compared to non-polar molecules. Examples of polar molecules include water (H₂O), ammonia (NH₃), and ethanol (C₂H₅OH).
Characteristics of Non-Polar Molecules
Non-polar molecules typically have weaker intermolecular forces, such as London dispersion forces, leading to lower boiling and melting points. Common examples include oxygen (O₂), methane (CH₄), and benzene (C₆H₆).
Understanding Dipole Moment in Polar Molecules
The dipole moment is a quantitative measure of the polarity of a molecule. It arises from the separation of charge within the molecule and is expressed in debyes (D). A larger dipole moment indicates a greater degree of polarity.
For a molecule to exhibit a dipole moment, it must have polar bonds that do not cancel each other out due to the molecular geometry. For instance, carbon dioxide (CO₂) has polar bonds, but its linear geometry causes the dipoles to cancel, resulting in a non-polar molecule overall.
Read also:Connie Kline The Remarkable Journey Of A True Icon
Factors Influencing Dipole Moment
- Electronegativity Difference: The greater the difference in electronegativity between atoms, the stronger the dipole moment.
- Molecular Geometry: The shape of the molecule determines whether the individual bond dipoles cancel out or add together.
The Role of Molecular Geometry in Polarity
Molecular geometry plays a pivotal role in determining whether a molecule is polar or non-polar. Even if a molecule contains polar bonds, its overall polarity depends on the arrangement of these bonds in space.
For example, water (H₂O) has a bent molecular shape, which prevents the dipoles from canceling out, making it a polar molecule. Conversely, carbon tetrachloride (CCl₄) has a tetrahedral geometry that allows the dipoles to cancel, resulting in a non-polar molecule.
Common Molecular Shapes and Their Polarity
- Linear: Non-polar if the electronegativities of the atoms are equal.
- Bent: Often polar due to uneven charge distribution.
- Tetrahedral: Non-polar if all surrounding atoms are identical.
Electronegativity and Its Impact on Polarity
Electronegativity is the ability of an atom to attract electrons within a covalent bond. The difference in electronegativity between atoms determines the polarity of the bond. If the difference is significant, the bond is polar; if it is negligible, the bond is non-polar.
Pauling's electronegativity scale is commonly used to quantify this property. For example, fluorine has the highest electronegativity (4.0), while francium has the lowest (0.7).
How Electronegativity Affects Molecular Polarity
In molecules like hydrogen fluoride (HF), the high electronegativity of fluorine creates a polar bond, making the molecule polar overall. In contrast, hydrogen gas (H₂) consists of two identical atoms with equal electronegativity, resulting in a non-polar molecule.
Examples of Polar and Non-Polar Substances
To better understand the concept of polarity, let’s examine some common examples of polar and non-polar substances.
Polar Substances
- Water (H₂O)
- Ammonia (NH₃)
- Ethanol (C₂H₅OH)
Non-Polar Substances
- Oxygen (O₂)
- Methane (CH₄)
- Benzene (C₆H₆)
Real-World Applications of Polar and Non-Polar Molecules
The distinction between polar and non-polar molecules has numerous practical applications across various fields.
In the pharmaceutical industry, understanding molecular polarity is essential for designing drugs that can effectively interact with biological targets. Polar molecules are often used in solvents for cleaning and dissolving substances, while non-polar molecules are utilized in lubricants and hydrophobic coatings.
Environmental Implications
Polar and non-polar molecules also play a role in environmental science. For instance, polar water molecules are crucial for the dissolution of pollutants, while non-polar hydrocarbons contribute to oil spills and environmental contamination.
Comparison Between Polar and Non-Polar Molecules
While both polar and non-polar molecules are essential in chemistry, they exhibit distinct characteristics that set them apart.
Polar molecules tend to have higher boiling and melting points due to stronger intermolecular forces, whereas non-polar molecules generally have lower boiling and melting points. Additionally, polar molecules are more likely to dissolve in water, while non-polar molecules are more soluble in organic solvents.
Key Differences
- Charge Distribution: Uneven in polar molecules, even in non-polar molecules.
- Intermolecular Forces: Stronger in polar molecules, weaker in non-polar molecules.
- Solubility: Polar molecules dissolve in water, non-polar molecules dissolve in organic solvents.
Solubility and Polarity: How They Relate
The principle "like dissolves like" is a fundamental concept in chemistry that explains the relationship between solubility and polarity. Polar solvents, such as water, dissolve polar solutes effectively, while non-polar solvents dissolve non-polar solutes.
This principle is critical in applications like cooking, where polar water is used to dissolve salt and sugar, and non-polar oil is used to dissolve fats and oils.
Exceptions to the Rule
Some molecules exhibit amphiphilic properties, meaning they have both polar and non-polar regions. These molecules, such as soaps and detergents, can dissolve in both water and oil, making them valuable in cleaning and emulsification processes.
Methods for Testing Molecular Polarity
Determining the polarity of a molecule can be achieved through various experimental techniques.
One common method involves measuring the dipole moment using infrared spectroscopy. Another approach is to assess the solubility of the substance in polar and non-polar solvents. Additionally, computational chemistry tools can predict molecular polarity based on theoretical models.
Challenges in Testing Polarity
While these methods are effective, they may not always provide definitive results, especially for complex molecules. Researchers must carefully analyze the data and consider multiple factors to ensure accurate conclusions.
Conclusion: Why Understanding Polarity Matters
In conclusion, the concept of polar vs non-polar molecules is a cornerstone of chemistry with far-reaching implications in science and industry. By understanding the factors influencing molecular polarity, such as electronegativity, molecular geometry, and dipole moment, we can better predict and manipulate the behavior of substances in various environments.
We invite you to explore further resources on this topic and share your thoughts in the comments section below. If you found this article helpful, consider sharing it with others who may benefit from this knowledge. Additionally, feel free to browse our other articles for more insights into the fascinating world of chemistry.
Data sources for this article include reputable scientific journals and textbooks, ensuring the accuracy and reliability of the information provided. Understanding molecular polarity is not just an academic pursuit; it is a practical tool that shapes our world in countless ways.